Our Clinical Trials
Advancing the Treatment of Cancer
A cancer clinical trial, also known as cancer research studies, are research studies that involves testing new types of treatment on people in the hopes of finding new and better ways to treat cancer. Hawaiian’s with cancer are living longer today because of advancements in cancer research and successful clinical trials that took place years before. Almost all medical treatment methods and medications commonly available today are because of clinical trials. The only way to know if a promising new treatment will be safe and effective is to have clinical trials and clinical trial volunteers make that possible.
Our cancer clinical trials in Hawaii are not just limited to testing new medications. Cancer clinical trials can be designed to find new ways to detect and diagnose cancer, treat cancer, prevent cancer, or make quality of life improvements to manage symptoms and side effects from cancer treatment in cancer patients.The types of new treatments being researched can include trying new surgical procedures or advanced chemotherapy techniques. Studies may also include trying a new combination of treatments and methods. Additionally, it may involve testing to determine if a treatment already being administered can be used to treat another form of cancer. Because of the different types of clinical trials available, clinical trials are always an option to consider when receiving cancer treatment.
Understanding the Clinical Trial Phases
The cancer clinical trials in Hawaii and those being conducted on the mainland are not all the same. There are different steps, or phases during a trial. Each phase has a specific purpose and is looking to answer different questions about the new treatment. As a potential clinical trial patient, it is important to become knowledgeable about the different trial phases before you decide to participate.
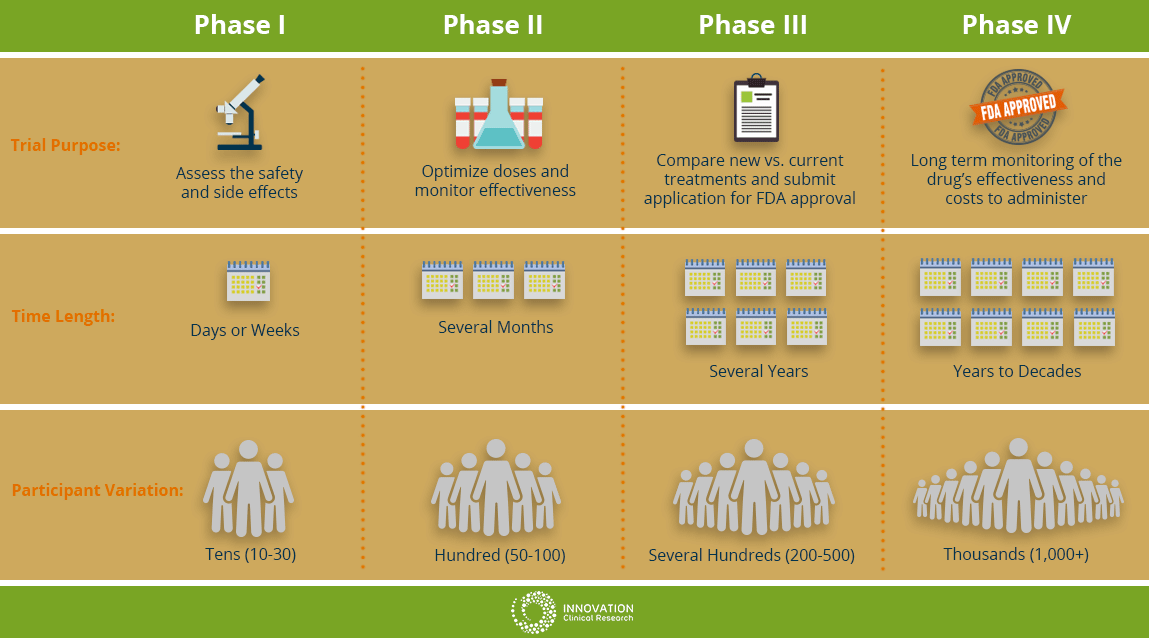
Phase I
This will be the first time the new drug or treatment is given to human volunteers. In this phase, doctors are determining the safety of the drug and optimizing the treatment dosage. They are also seeking the best way to give the new medication; by mouth, injection or IV drip. Additionally, the researchers are observing for and identify any side effects of the new treatment on the patient. This phase is usually limited to a small group of cancer patients.
Phase II
In this phase, the new treatment is on people with a specific type of cancer to see how the cancer responds to the new treatment. The researchers might be observing if there is any effect on the cancer such as slowing cancer cell growth of any shrinking in tumor size. The safety of the patients will continue to be monitored as well observing for any side effects.
Phase III
In this phase of cancer clinical trials, researchers evaluate the difference between the treatment currently used (standard treatment) versus the new treatment being studied to see which is better. In this phase, patients will be randomly assigned into groups where some patients will receive the new treatment and some will be placed in the standard treatment group (or control group). This is known as randomization. This will help researchers compare side effects and assess the benefits and risks of both treatments to see which treatment performs better. At least several hundred patients to thousands are involved and these trials can take place nationwide and around the world.
Phase IV
At this stage, the drug has obtained approval by the FDA and has been found to be safe and effective for people. During this phase, the drug will continue to be extensively studied and evaluated for long term effectiveness and safety over time. The costs associated with the proposed drug will also be monitored.
The Different Clinical Trial Phases
Considering the Benefits and Risks
Before you consider joining any cancer clinical trials in Hawaii or starting any new treatment, it is always important to look at the potential benefits and risks involved. Being informed on what the treatment benefits and possible risks are will help you make the best decision for your medical situation.
Potential Benefits
- You may be one of the first to benefit from a new treatment or procedure that is not widely available
- You will be closely monitored and receive extra care because of more frequent doctor visits and testing needed to monitor your progress and side effects that may occur
- You will play a more proactive role in your own healthcare because you are exploring other treatment options and weighing the benefits and risks involved
- Your participation will help future cancer patients in the discovery of new treatments and preventions methods
- Even if the new treatment proves not to be better than the treatment commonly used, the information learned pushes researchers to develop new and better treatments methods
Potential Risks
- There is no guarantee the new treatment you receive will be better than standard treatment
- There may be unexpected side effects
- Patients may experience side effects that are worse than if you received standard care
- The new treatment being offered may work for some but may not benefit you
- Extra visits and tests may be uncomfortable
- Insurance companies may not cover all patient costs
- Extra time needed to visit doctors for extra visits, additional tests, and hospital stays possibly resulting in increased transportation and childcare costs
Participating in Clinical Trials
Deciding whether to participate in a cancer clinical trial is a decision you need to carefully consider with your family members and doctors. It is important to make sure all your questions are answered before making the key decision to participate in a clinical trial. Participation may possibly enhance your overall medical treatment; however, patients must look at the potential benefits and risks associated with any form of cancer treatment. Ultimately, your decision to participate will help future cancer patients and help advance cancer research studies.
Am I Eligible to Participate in a Clinical Trial?
Every clinical trial has specific guidelines stating who can join known as eligibility criteria. Characteristics that participants must have are determined by strict guidelines listed in the protocol. Eligibility criteria is different for every clinical trial because every trial is unique.
Before participating in clinical trials, you will receive health screenings and medical tests to make sure the trial is a good fit for you. Patients that do not meet eligibility criteria may not participate in the clinical trial, but your doctor can see if there are other research studies you are eligible for. Eligibility criteria can include:
- Age
- Gender
- Type and stage of cancer
- Prior cancer treatment history
- Other present medical conditions
- Overall health status
Clinical Trial Research Protocols
Every clinical trial has a detailed action plan that doctors must follow known as the protocol. The protocol will state what will be done in the research study and why. This is prepared by the lead doctor in charge of the clinical trial, or principal investigator. These same protocols and guidelines must be followed by any doctor participating in the clinical trial. The protocols can include:
- What the clinical trial is for
- Who may participate (eligibility criteria)
- How many participants are needed
- The type of drugs being used, dosage levels, lab work, tests and drug schedule
- How long the trial will last
- The type of participant information researchers are going to collect
Patient Safety Protocols
Every clinical trial conducted in the United States requires there to be an Institutional Review Board (IRB) to approve and monitor the clinical trial. The IRB monitors the clinical trial to make sure the research study being conducted is safe and ethical for the clinical trial volunteers. Members of the IRB usually consist of an independent group of doctors, scientists, nurses, lawyers, patient advocates, and people from the local community. They review the protocol before the trial begins and continually monitor the trial from beginning to end. IRB group members can make changes to the protocol, or end a trial, in the interest of patient safety. The IRB is monitored by the U.S. Food and Drug Administration (FDA) who can oversee and review any clinical trial at any time they see fit.
Costs for Participating in Clinical Trials
Your health insurance should cover your routine doctor visits, hospitalization, lab work and any treatment associated with treating your cancer. The clinical trial sponsor will cover the costs of the study drug, and any extra lab tests or procedures that are performed strictly for the study. You should discuss with your doctor and care team before you decide to participate how much your health insurance will cover as every health plan is different for every patient.
Furthermore, you also need to consider the additional time you will spend visiting the clinic where the trial is taking place and expenses if you do not live close by. You may need to factor in extra transportation costs and childcare expenses as well. Some clinical trial sponsors may provide financial assistance with additional trial related costs. Your care team will discuss this with you during the informed consent process.
Understanding Patient Informed Consent
Before you participate in a clinical trial, you must give your informed consent as part of the process. This is where you learn details about the trial from your doctor or nurse before deciding whether to participate or not. They will explain the trial details to you and be available to answer any questions and address any concerns you may have. Part of the discussion may include:
- The purpose of the trial or protocol
- Tests and procedures that will be used
- Possible benefits and risks
- How treatment will be administered
- Eligibility criteria
- Other treatment options available
- Who covers the cost of the trials and possible extra costs to you
- Patient information they will collect
- And your rights as a patient
Before you agree to participate, make sure to ask questions and feel satisfied that all your questions have been answered. It may help to ask trusted family and friends to help you with this process. If you agree to participate, you will be asked to sign the informed consent form. Once the clinical trial begins, if there are any changes or new information becomes available about the study, you will be told what those changes are. Even though you sign the informed consent form and agree to participate, you are free to leave the study at any time you choose for any reason.
Clinical Trials Frequently Asked Questions
Will I be a “guinea pig” if I participate in a clinical trial?
This is a common concern among patients when they consider participating in clinical trials. Patients are concerned that they will not know what is going on and will be at the mercy of the researchers. Fortunately, this not true. Because of the “Informed Consent” process, you will be made fully aware of what will happen during the trial and know what to expect. Even if changes occur during the trial, you will be informed of those changes as well. You will receive excellent care because of the extra attention by your doctor and research team to make sure your health is not being negatively affected in any way. Your health and well-being is always a top priority.
How do I know if I am able to participate in a clinical trial?
Every clinical trial has a set of guidelines that determine what characteristics a participant must have in order to participate known as Eligibility Criteria. This determines who can participate in a trial and who cannot. Your doctor will go over the criteria to make sure the trial is a good fit for you based on your health history.
Do I have to be a Hawaii Cancer Care patient to participate in a clinical trial?
Hawaii Cancer Care welcomes all patients who are considering joining a cancer clinical trial. You do not have to be a current patient with Hawaii Cancer Care. Our care team will help you with the transition process. If you do not live in Hawaii and need assistance to find housing to receive treatment, our care team can assist you with this as well.
What happens before a clinical trial begins?
Before any clinical trial begins, there were many years of research in a lab to develop and understand the effects of the new treatment on cancer cells. Researchers are looking to see how cancer cells (in a dish) or animals with cancer respond to the new treatment. However, even with all the testing and information being collected in the lab, the drug or treatment may perform or react differently in humans. For instance, a cancer treatment that showed promise in mice may not have the same results in people. Before clinical trials can begin, the U.S. Food and Drug Administration (FDA) must grant permission for its use.
Who is in charge of the clinical trial?
Every clinical trial has a medical doctor, also known as the principal investigator, who is in charge and the lead researcher of the trial. The principal investigator also prepares an action plan for the trial known as the protocol. The principal investigator works with a research team of other doctors, nurses, and other health professionals to make sure the protocol is being followed and the trial is running smoothly.
Are clinical trials only for those who don’t have any other treatment options?
It is a myth that clinical trials are only for people with advanced stages of cancer with no other options for treatment. There are trials available for all stages of cancer. Many newly diagnosed cancer patients choose to participate in clinical trials as their first cancer treatment.
Are cancer clinical trials only to study new medications or drugs?
There are other types of cancer clinical trials and they don’t always just involve testing drugs. There are trials available for cancer patients, cancer survivors, or those who are at high risk for developing cancer. Clinical trials should always be considered as a treatment option.
- Treatment trials explore new experimental treatment, such as a new drug, surgery techniques, or radiation therapy techniques. Treatment methods can be combined or a treatment commonly used may be tested in a different way.
- Prevention trials look to prevent cancer in people who never had cancer but are at high risk or have had cancer and trying to prevent recurrence or a new cancer from developing. This can involve lifestyle changes or taking medications, vitamins, minerals or dietary supplements.
- Screening trials are designed to find better ways to detect a disease or condition in its early stages.
- Diagnostic trials aim to develop tests or procedures to diagnose a disease or condition better.
- Quality of life trials (or supportive care trials) explore ways to improve the comfort level and quality of life for patients, especially those who have had side effects, during or after treatment has concluded.
What are placebos and will I be given one if I participate?
Placebos, or “sugar pills” are pills that have no effect on the patient if it is given to them. Cancer clinical trial participants are almost never given a placebo if there is a treatment option available. Usually, a placebo is used in conjunction with the standard treatment to compare it to the new treatment (combined with the standard treatment) being studied, or if there is no standard treatment available. If it is possible a placebo will be used in a trial, the doctor will let you know during the informed consent process.
Can I leave a clinical trial at any time?
You can leave a study at any time for any reason. You can discuss other treatment options available to you with your doctor and still expect to receive a high level of care.
Can I continue to see my regular doctor?
Several of our doctors at Hawaii Cancer Care are principal investigators for cancer clinical trials who will oversee and work closely with you. You may still be able to visit with your regular doctor while you are participating in a cancer clinical trial if you are currently a patient of Hawaii Cancer Care.
Will I have to travel to the mainland if I join a clinical trial?
Our clinical trials take place at Hawaii Cancer Care clinics, so traveling to the mainland is not necessary. If there is not a trial available for you through Innovation Clinical Research, your doctor can help you find one here in Hawaii. It is important to be home with family and friends close by while you are on your journey to healing.
Can a clinical trial end while I am part of the study?
A clinical trial can change or end while you are participating for different reasons. The doctors or IRB may stop a trial because they are finding participants are experiencing serious or harmful side effects. It can also end if researchers are finding results that are better than expected and would like to submit the drug or treatment for approval to the FDA (Food and Drug Administration) for release as a new standard treatment. Patients affected by any trial stoppage will be offered additional treatment options.
Questions to Ask Your Doctor
If you are considering participating in a cancer clinical trial, it is important to know as much as possible about the study before you agree to participate. The informed consent process should provide answers to many of your questions. However, you should feel comfortable asking your doctor and research team any other questions you may have and be able to receive further clarification on something that was already explained.
Here are some questions you may want to ask and discuss with your healthcare team. Being well informed is very important in the decision-making process of whether to participate in a cancer clinical trial.
Regarding the Clinical Trial
- What is the purpose of the clinical trial?
- Am I eligible to participate in the trial?
- Why do researchers feel this new treatment being tested is better than what is already being offered?
- Has it been tested before?
- How is it not better than treatment being used now?
- Where will the trial take place?
- Can I speak to someone that is currently participating or has already participated in the trial?
- How long will the trial last?
- Why should I participate in a cancer clinical trial?
- Can I stop at any time?
Health and Medical Treatment Considerations
- What possible side effects, risks and benefits are expected? How much will it impact my health?
- What types of tests and procedures should I expect during the trial?
- How often will I need to go to the doctor’s office or hospital?
- Will I need to be hospitalized? If yes, how often and for how long?
- Who will be in charge of my care during the trial?
- Can I still see my own doctor?
- How will I know the treatment is working?
- If I am injured during the trial, what options will I have available to me?
- Who can I speak to if I have any questions during and after the trial?
- What are ways my daily life can be affected while on the study?
- What type of support and resources will my caregivers and I have access to during the trial?
After the Clinical Trial is Completed
- Will I find out what is learned or discovered after the study is complete?
- Is there long-term care or follow-up provided after the trial is over?
Cost Considerations
- Who will cover my costs for participating in the trial?
- Will my health insurance cover my costs?
- Will I need to pay for anything?
- What will I be responsible for or is there anything I will need to do?
- Will I be reimbursed if I have to pay for anything out of pocket?